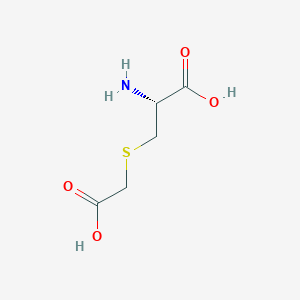
Mucolytic
Adult: As adjunctive therapy in respiratory tract disorders characterised by excessive, viscous mucus secretions (including COPD): Initially, 2,250 mg daily in divided doses, may be decreased to 1,500 mg daily in divided doses when a satisfactory response is obtained.
Child: 2-5 years Usual dose: 62.5-125 mg 4 times daily or 200 mg daily in 2 divided doses; 6-12 years Usual dose: 100 mg or 250 mg tid. Dosage recommendations may vary among individual products and between countries (refer to detailed product guidelines).
Child: 2-5 years Usual dose: 62.5-125 mg 4 times daily or 200 mg daily in 2 divided doses; 6-12 years Usual dose: 100 mg or 250 mg tid. Dosage recommendations may vary among individual products and between countries (refer to detailed product guidelines).




 Đăng xuất
Đăng xuất