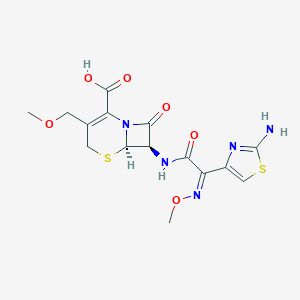
Pharyngitis, Tonsillitis
Adult: Dosage is expressed in terms of the active cefpodoxime moiety: 100 mg 12 hourly for 5-10 days. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: 2 months to 12 years As susp: 4-5 mg/kg 12 hourly for 5-10 days. Max: 200 mg daily. >12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: 2 months to 12 years As susp: 4-5 mg/kg 12 hourly for 5-10 days. Max: 200 mg daily. >12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Oral
Skin and skin structure infections
Adult: Dosage is expressed in terms of the active cefpodoxime moiety: 400 mg 12 hourly for 7-14 days. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Oral
Acute otitis media
Adult: Dosage is expressed in terms of the active cefpodoxime moiety: 200 mg 12 hourly, 5-7 days for mild to moderate infection and 10 days for severe infection. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: 2 months to 12 years As susp: 4-5 mg/kg 12 hourly for 5 days. Max: 200-400 mg daily. >12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: 2 months to 12 years As susp: 4-5 mg/kg 12 hourly for 5 days. Max: 200-400 mg daily. >12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Oral
Uncomplicated gonorrhoea
Adult: Dosage is expressed in terms of the active cefpodoxime moiety: 200 mg as a single dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Oral
Acute bacterial sinusitis, Acute maxillary sinusitis
Adult: Dosage is expressed in terms of the active cefpodoxime moiety: 200 mg 12 hourly for 10 days. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: 2 months to 12 years As susp: 4-5 mg/kg 12 hourly for 10 days. Max: 200-400 mg daily. >12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: 2 months to 12 years As susp: 4-5 mg/kg 12 hourly for 10 days. Max: 200-400 mg daily. >12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Oral
Acute bacterial exacerbation of chronic bronchitis
Adult: Dosage is expressed in terms of the active cefpodoxime moiety: 200 mg 12 hourly for 10 days. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Oral
Uncomplicated urinary tract infections
Adult: Dosage is expressed in terms of the active cefpodoxime moiety: 100 mg 12 hourly for 7 days. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Oral
Community-acquired pneumonia
Adult: Dosage is expressed in terms of the active cefpodoxime moiety: 200 mg 12 hourly for 14 days. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: Infants (15 days) to 11 years As susp: 4 mg/kg 12 hourly. Max: 200 mg daily. ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.
Child: Dosage is expressed in terms of the active cefpodoxime moiety: Infants (15 days) to 11 years As susp: 4 mg/kg 12 hourly. Max: 200 mg daily. ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to country- or product-specific guidelines.




 Đăng xuất
Đăng xuất