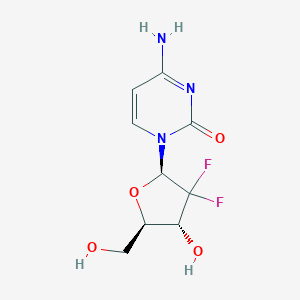
Cancer, pancreatic
Adult: Patient w/ locally advanced or metastatic cases: 1,000 mg/m2 once wkly via infusion over 30 min for 7 wk followed by 1 wk rest, then once wkly for 3 consecutive wk out of every 4 wk cycle. Dosage may reduce w/ each cycle or w/in a cycle based on toxicity.
Intravenous
Breast cancer
Adult: Patient w/ unresectable, locally recurrent, or metastatic cases: Combination therapy w/ paclitaxel: 1,250 mg/m2 via infusion over 30 min on days 1 and 8 of a 21-day cycle. Dosage may reduce w/ each cycle or w/in a cycle based on toxicity. Patients should have absolute granulocyte count of ≥1,500 x 106/L prior to initiation of therapy.
Intravenous
Bladder cancer
Adult: Patient w/ locally advanced or metastatic cases: Combination therapy w/ cisplatin: 1,000 mg/m2 via infusion over 30 min on days 1, 8 and 15 of a 28-day cycle. This 4-wk cycle is then repeated. Dosage may reduce w/ each cycle or w/in a cycle based on toxicity.
Intravenous
Non-small cell lung cancer
Adult: Patient w/ locally advanced or metastatic cases: Monotherapy: 1,000 mg/m2 once wkly via infusion over 30 min for 3 wk followed by 1 wk rest for locally advanced or metastatic cases. This 4-wk cycle is then repeated. Combination therapy w/ cisplatin: 1,000 mg/m2 via infusion over 30 min on days 1, 8, and 15 of a 28-day cycle. Alternatively, 1,250 mg/m2 via infusion over 30 min on days 1 and 8 of a 21-day cycle. Dosage may reduce w/ each cycle or w/in a cycle based on toxicity.
Intravenous
Ovarian cancer
Adult: Patient w/ locally advanced or metastatic epithelial cases: Combination therapy w/ carboplatin: 1,000 mg/m2 via infusion over 30 min on days 1 and 8 of a 21-day cycle. Dosage may reduce w/ each cycle or w/in a cycle based on toxicity.




 Đăng xuất
Đăng xuất